General Formula of Alkane
The paraffins are major constituents of natural gas and petroleum. Paraffins containing fewer than 5 carbon atoms per molecule are usually gaseous at room temperature those having 5 to 15 carbon atoms are.
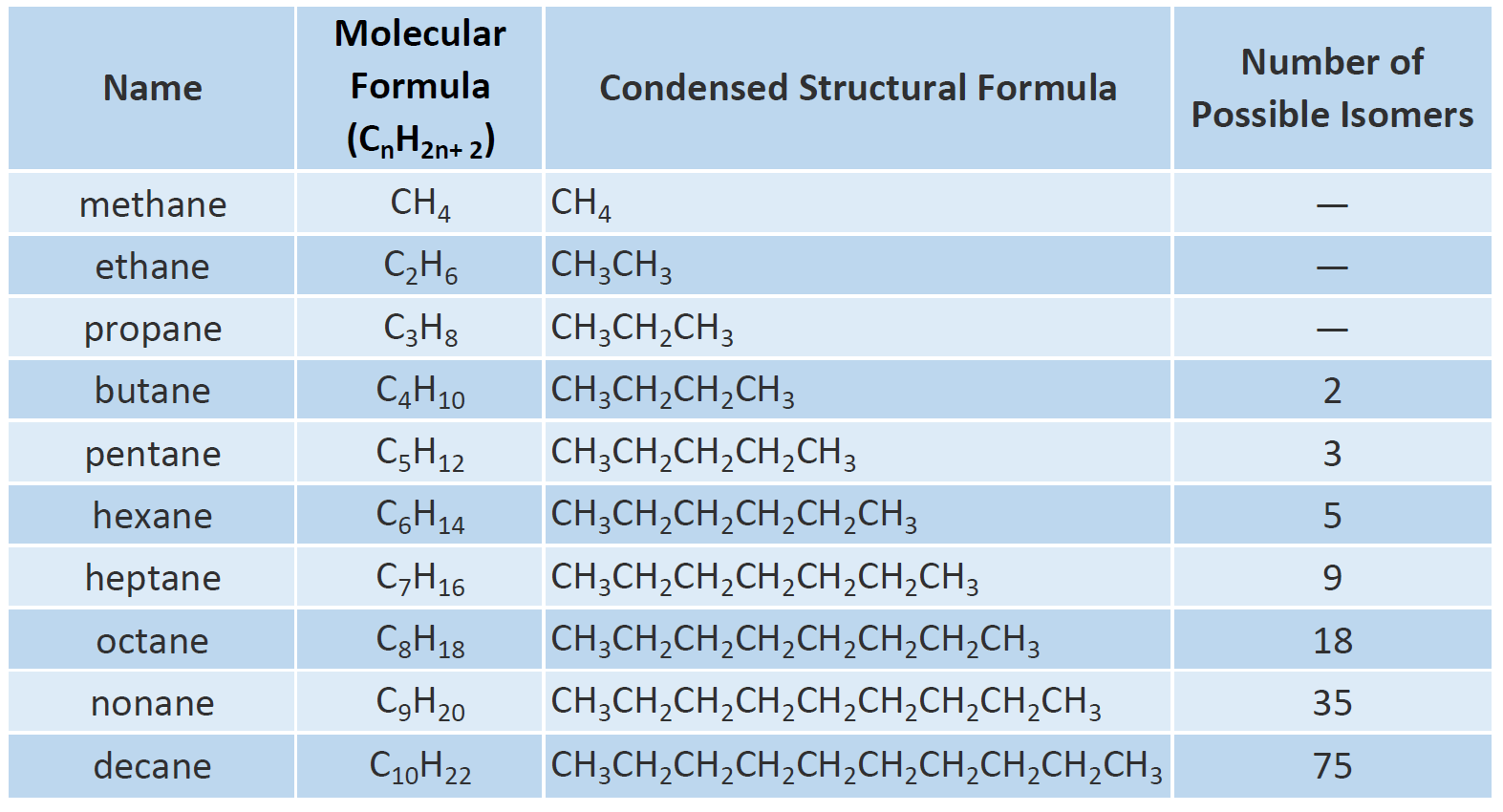
Ch105 Chapter 7 Alkanes And Halogenated Hydrocarbons Chemistry
General Properties of Alkenes.
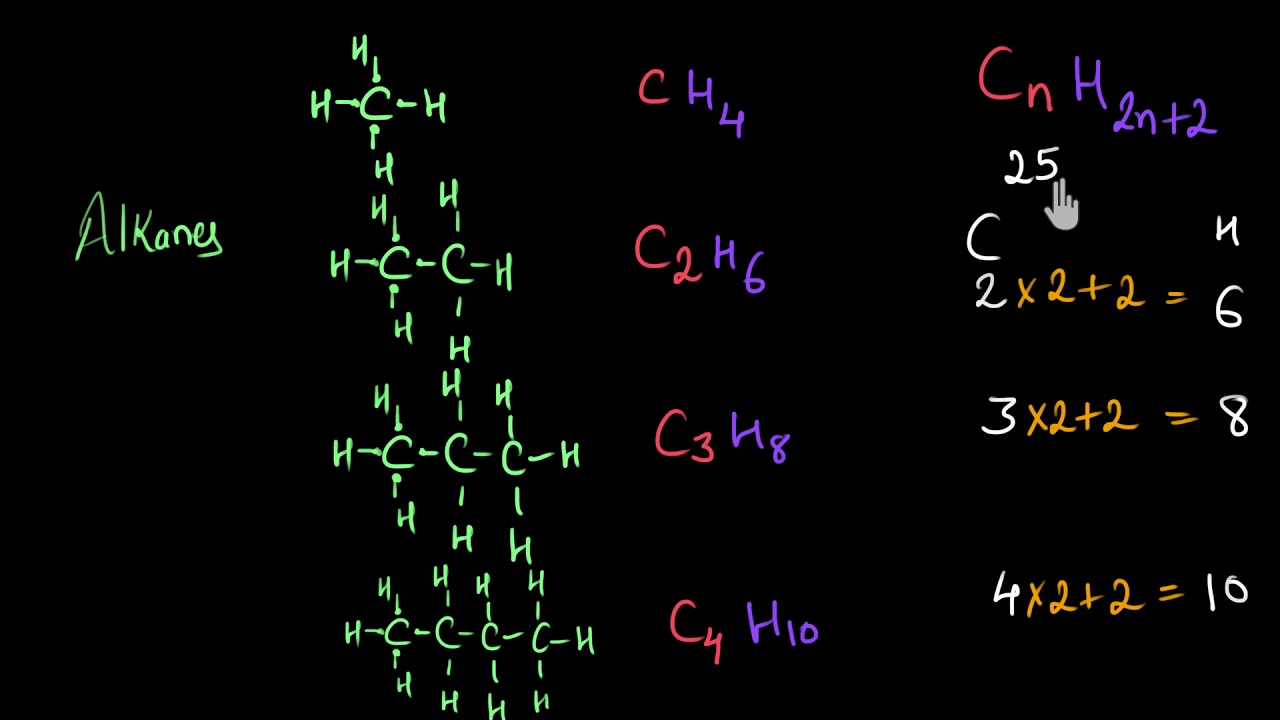
. There is no limit of how much carbons can be tied together. Remember that carbon must have four bonds oxygen must have two. A sulfonic acid or sulphonic acid refers to a member of the class of organosulfur compounds with the general formula RSO 2 OH where R is an organic alkyl or aryl group and the SO 2 OH group a sulfonyl hydroxide.
F CH3 Na OČCH3 CHCO2H Use the wedgehash bond tools to indicate stereochemistry where it exists. Paraffin hydrocarbon also called alkane any of the saturated hydrocarbons having the general formula CnH2n2 C being a carbon atom H a hydrogen atom and n an integer. Methane is the simplest of saturated hydrocarbons with a chemical formula CH 4.
What is the general formula for this series. Problem 132 Write structur es of dif fer ent isomeric alkyl gr oups corr esponding to the molecular for mula. When naming organic acids.
It consists of four hydrogen atoms and one carbon atom and is the simplest alkane. EDTA Polyacrylamide Carbomer Aminomethyl Propanol PEG-100 Stearate Ammonium Polyacrylate Titanium Dioxide C13-14 Alkane 12-Hexanediol. It also can be more than 10 carbon atoms.
Nomenclature of substituted alkanes can further be understood by considering the following problem. General Structure or Formula. The general formula for alkanes is CnH2n 2.
Draw a structural formula for the substitution product of the reaction shown below. The branched groups must be listed before the name of the main chain in alphabetical order ignoring ditritetra. News Corp is a global diversified media and information services company focused on creating and distributing authoritative and engaging content and other products and services.
Physical state The members containing two or four carbon atoms are gases five to seventeen liquids eighteen onwards solids at room temperature and they burn in air with a luminous smoky flame. Fast absorbing formula will not clog the pores of the skin. Fights the 7 signs of ageing for a glowing and fabulous skin.
Alcohols have the same general formula as alkanes but the structure of alcohol functional group is textOH called the hydroxyl group. Hydrocarbon any of a class of organic chemical compounds composed only of the elements carbon C and hydrogen H. They serve as fuels.
If more than one stereoisomer of product is formed draw both. General formula for alkyl groups is CnH2n1 Unit 12. Let us recall the general rules for nomenclature already discussed in Unit 12.
Hydrocarbons are the principal constituents of petroleum and natural gas. Visit general health services. When natural methane reaches the surface of the atmosphere is called atmospheric methane and can be found under the seafloor as well as below the ground.
Some types of oils and waxes are the examples of alkanes with many carbon atoms number. The simplest alkane is methane with the formula ofCH4. The general formula for the alkanes is C n H 2n 2 where n is the number of carbon atoms in the molecule.
Winter Flu Jab Service. Elemental analysis is a process where a sample of some material eg soil waste or drinking water bodily fluids minerals chemical compounds is analyzed for its elemental and sometimes isotopic composition. As a substituent it is known as a sulfo groupA sulfonic acid can be thought of as sulfuric acid with one hydroxyl group replaced by an organic substituent.
If the molecule is an alkane the branched group must be on the carbon with the lowest possible number. Its molecules contain six carbon atoms. Citation needed Elemental analysis can be qualitative determining what elements are present and it can be quantitative determining how much of each are present.
Density Alkenes are lighter than water. The carbon atoms join together to form the framework of the compound and the hydrogen atoms attach to them in many different configurations. Solubility Alkenes are insoluble in water and soluble in organic solvents such as benzene etc.
The most common alcohol known as ethanol is used in alcoholic drinks fuel gasoline a preservative for biological specimens and a solvent for paints and drugs. Separate multiple products using the sign from the drop-down menu. Hexane is an alkane.

Alkanes Alkenes And Alkynes General Molecular Formula Chemistry Khan Academy Youtube
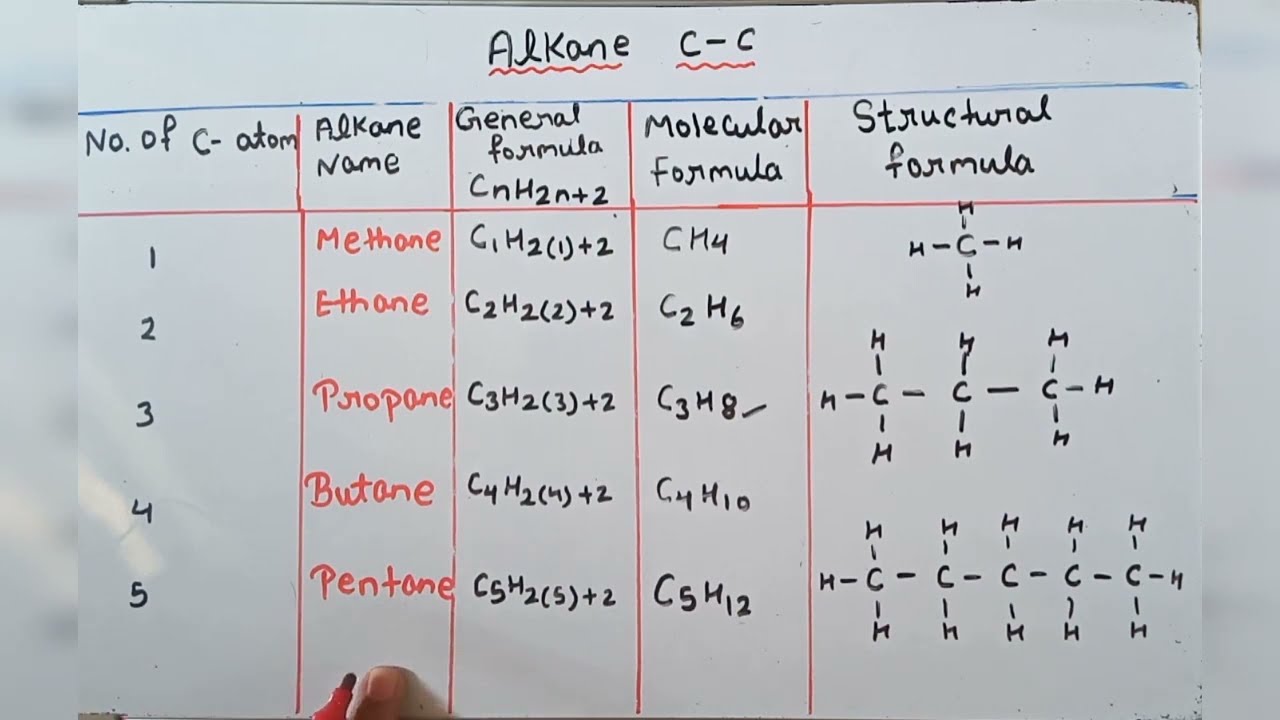
Alkane Molecular Structural General Formula Youtube

Question Video Applying The General Formula For Alkane Chemical Formulas Nagwa

What Is The Formula Of Alkane Quora
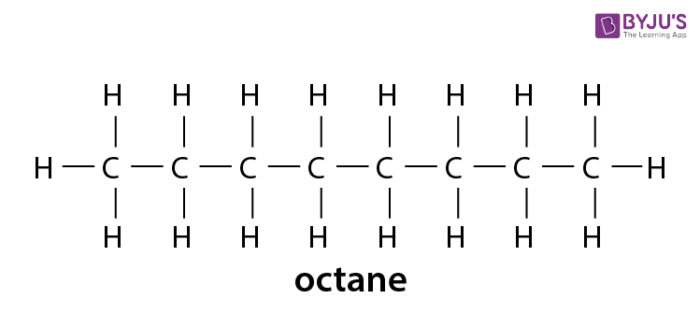
Alkanes Formula Definition Structure Properties List Of Alkanes Videos Examples And Faqs Of Alkanes

Class 10 General Formula Of Alkane Alkene Alkyne Tx Academy Youtube
0 Response to "General Formula of Alkane"
Post a Comment